

Potentiostats/Galvanostats for electrochemical tests
These instruments are used for advanced electrochemical tests to evaluate the corrosion behaviour of bare or coated metallic materials in various liquid corrosive environments. The tests may be potentiodynamic/galvanodynamic or electrochemical impedance (not all instruments can perform this measurement). In the case of potentiodynamic tests, the instruments generate electrical potentials or electric currents with well-defined laws and at the same time read signals from a 3-electrode electrochemical cell (Ag/AgCl reference electrode, working electrode/sample electrode and Pt counter-electrode; all immersed in the test solution). What is measured are potentiodynamic or galvanodynamic curves in which the imposed potential vs. the read electric current, or the imposed current vs. the imposed potential respectively, is related. These tests are useful for assessing the barrier effect provided by coatings or surface deposits or for assessing the degree of activity of the material in the test environment considered (assessment of whether the material is active or passive in the test environment). Potentiodynamic/galvanodynamic tests can be carried out in appropriate electrochemical cells whose task is to control the temperature or to prevent, in the case of passive materials, the production of crevice corrosion during the test (Avesta cell).
Tests can also be carried out by keeping the potential or current at a constant value and thus assessing the response of the material (potentiostatic or galvanostatic tests). The laboratory is also equipped with a multi-channel instrument to carry out several tests simultaneously.
Some instruments are equipped with modules for electrochemical impedance assessment (EIS). This measurement is usually carried out by varying the potential close to the corrosion potential of the material in a sinusoidal manner, with low perturbation values and a variable oscillation frequency. In this way, it is possible to assess the extent of the protective barrier provided by a coating or film of passivity, as well as its stability over time and the degree of defect. The assessment is usually made by evaluating Bode and Nyquist diagrams of the detected impedance.
Tests that can be performed with such instruments are:
Potentiodynamic/galvanodynamic tests to evaluate the barrier effect of deposits/coatings or to assess the material's susceptibility to corrosion. The tests can be carried out at room temperature and at high temperature in different corrosive solutions;
Potentiostatic/galvanostatic tests. Tests can be performed at room temperature and at high temperature in different corrosive solutions;
Electrochemical impedance tests in different solutions for varying immersion times;
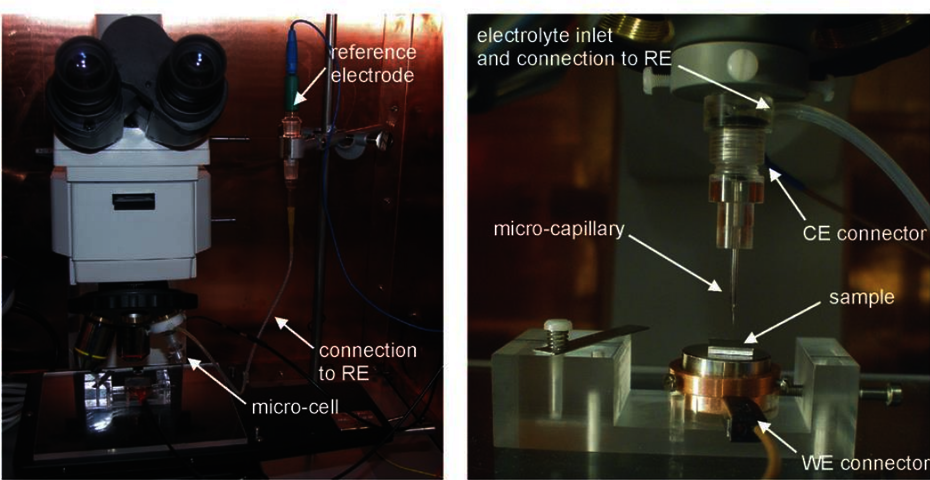
Electrochemical microcell
The electrochemical microcell is a very special instrument that allows the electrochemical tests described above to be performed on a very small (sub-millimetre/micrometre) scale. In this case, the electrochemical cell consists of self-produced glass capillaries with internal diameters ranging from 50 micrometres to 0.8mm. In this case, the area exposed to the corrosive fluid, with which the cell containing the glass capillary is filled, is very small such that very localised measurements can be taken. The instrument is very useful for understanding micro-corrosive mechanisms occurring in materials, such as mechanisms in the vicinity of particles or phases constituting the material. the technique is also useful for assessing the corrosive behaviour of very small areas such as thermally altered zones in weld seams. The electrochemical cell, in this case, is mounted on an optical microscope revolver and the capillary is centred with the objective. This allows the user to first view the analysis zone with the instrument's optics and then, by rotating the revolver, to perform the analysis in the desired zone. The instrument is then connected with a potentiostat with very high sensitivity in both potential and current.
The potentiodynamic/galvanostat tests that can be performed are:
Micro-corrosion tests on phases and precipitates visible under an optical microscope;
evaluation of the corrosion behaviour of very small areas;
